美国当地时间11月19日,UPI华盛顿报道: 本周四,美国食品药品监督管理局(FDA)正式批准了转基因三文鱼在美国境内的销售。过去20年里,AquaBounty 公司一直为他们的三文鱼产品寻求FDA的批准。
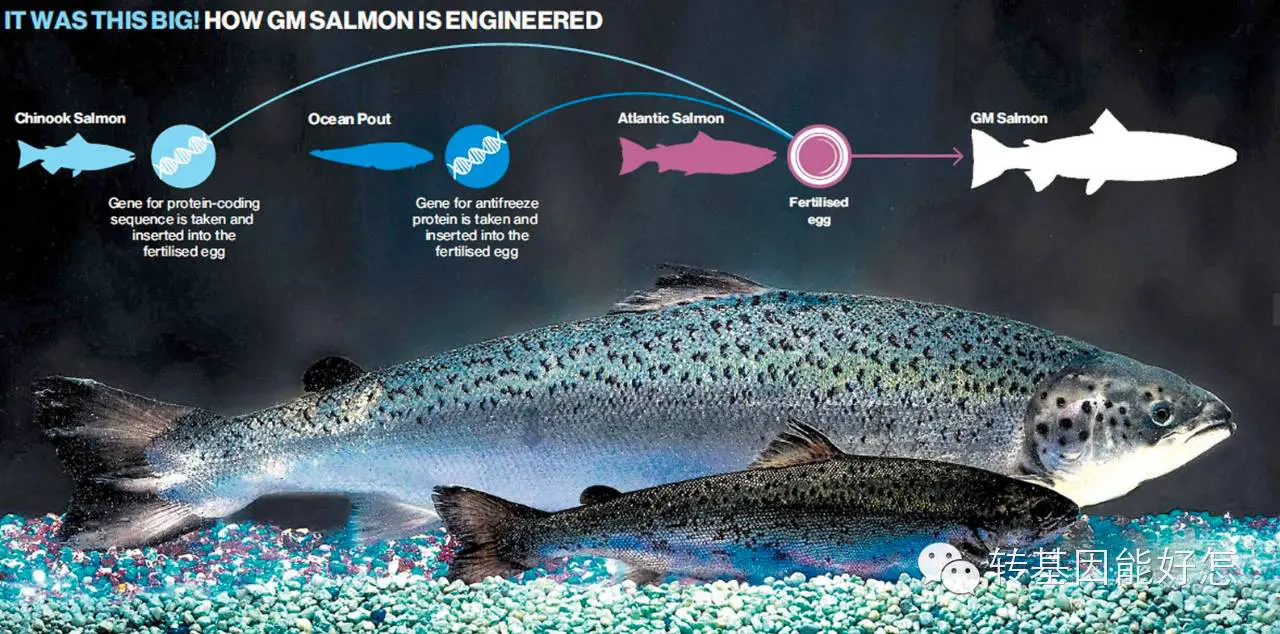
转基因三文鱼生长速度为普通三文鱼2倍
经过详细而严格的科学审查,FDA得出结论认为:这种具备养殖优势的三文鱼在食用安全性和营养方面都和非转基因的大西洋三文鱼一致。上述结论出自FDA刚刚发布的新闻。
尽管没有法律要求转基因三文鱼或者其他包含这种三文鱼的产品进行转基因标识,但FDA发布了两份文件来指导商家如何告知消费者产品中存在转基因成分。( 译者注:美国实行转基因食品自愿标识制度,中国实行定性强制标识制度)
FDA提醒商家:“如果食物或食品中的某些成分是来自基因工程的原料,消费者会想要知道这一点。”
FDA食品标签标准部门主管Felicia Billingslea补充到:“这两份指导性文件阐释了FDA的最佳考虑,即如何让消费者便利地获知一种食品是否由基因工程技术生产。”
据了解,美国AquaBounty公司此前已经获得了加拿大环保部的许可,获准在位于爱德华王子岛的养殖场孵化转基因三文鱼卵。这些鱼苗将在加拿大境内繁育,然后转运到巴拿马的陆上网箱养殖场来育肥。( 译者注:转基因三文鱼的生产目前仅限在指定的2家养殖场,这2家养殖场均与自然水系隔离。)经过基因改良,这种转基因三文鱼的生长速度是非转基因养殖三文鱼的两倍。
这家公司介绍,转基因三文鱼要在美国市场上市还需要至少2年(译者注:转基因三文鱼的生长周期为18个月),成品上市时,应该不会做转基因标识。
“当你是第一家,并且是唯一一家转基因三文鱼生产商时,标注转基因不是一个明智的商业决定”,AquaBounty 公司首席执行官 Ron Stotish 向《华盛顿邮报》表示,“我们希望把这种三文鱼标注为优质(转基因)产品,但实际操作中应该还是会用‘大西洋三文鱼’来作为商品名。”
不过,无论标识与否,这种转基因三文鱼的市场化之路看起来并不容易。连锁超市Whole Foods 和Trader Joe's 都已声明不会销售转基因三文鱼。
尽管科学界已经对转基因食品和转基因作物的绝大部分健康考量做了评估,但一些环保组织认为,仍然存在太多不可预知和未被研究过的风险。批评者对转基因鱼可能进入野外环境的生态风险表示担忧。
在批准转基因食品前的公众讨论期,FDA为公众提供了表达关切声音的机会。
注:原报道记者 Brooks Hays ,项栋梁翻译。转载请注明出处。
http://www.upi.com/Science_News/2015/11/19/FDA-okays-GM-salmon-for-sale-in-the-United-States/5991447949495/
FDA okays GM salmon for sale in the United States
WASHINGTON, Nov. 19 (UPI) ——On Thursday, the U.S. Food and Drug Administration approved a bid to sell genetically modified salmon in the United States. The company AquaBounty has sought FDA approval for their salmon products for the last 20 years.
"After an exhaustive and rigorous scientific review, FDA has arrived at the decision that AquAdvantage salmon is as safe to eat as any non-genetically engineered (GE) Atlantic salmon, and also as nutritious," the agencywrote in a news release.
Though no law will require GM salmon, or products containing GM salmon, to be labeled as such, the FDA issued two documents guiding manufacturers on how to inform their customers of the presence of genetically modified ingredients.
"[Customers] want to know whether their food or any ingredients in their food is derived from genetically engineered sources," the FDA warned.
"Both guidance documents explain FDA's best thinking on how to make it easy for consumers to know whether a food was produced using genetic engineering or not," added Felicia Billingslea, director of FDA's Division of Food Labeling and Standards.
AquaBounty, a U.S. company, has alreadygained permission from environmental officials in Canada to harvest GM Atlantic salmon fish eggs at its facilities in Prince Edward Island. The fingerlings are reared in Canada and then shipped to Panama to fatten up in above-ground tanks. The fish are modified to grow twice as fast as unmodified farm-raised salmon.
The company says it will be at least two years before its fish hits U.S. markets. When it does, it likely won't be labeled.
"When you're the first and only, labeling is a dangerous decision," AquaBounty CEO Ron Stotish told The Washington Post."We'd like to label it as a premium product, but we'll probably introduce it as 'Atlantic salmon.'"
Labeled or not, the salmon may have a tough time getting stocked. Grocery chains Whole Foods and Trader Joe's have already pledged not to carry GM salmon.
The scientific community has dismissed most health concerns about consuming genetically modified organisms, or GMOs, but some environmental groups suggest too many risks remain unknown and unstudied. Critics have expressed worries over the environmental and ecological consequences of GM fish should they escape into the wild.
The FDA will offer the public a chance to voice those concerns during the public comment period of their pending approval.

转基因能好怎(微信号:zjynhz)
了解它,才不会害怕






